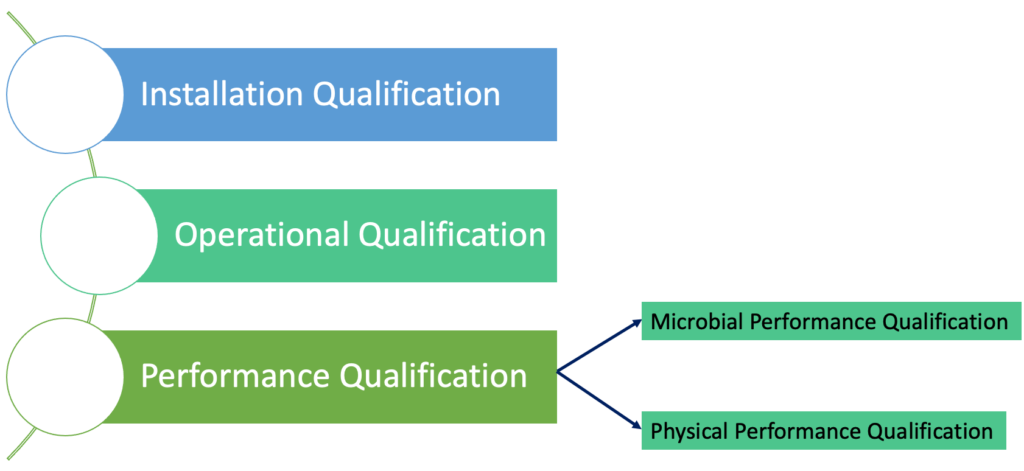
ISO 11135:1994, Medical devices - Validation and routine control of ethylene oxide sterilization: ISO TC 198/WG 1: Amazon.com: Books

ISO 11135:2014 - Understanding the changes | Take a look at our recent webinar in which Jenni Tranter and Richard Cowman discuss the changes to ISO 11135:2014. Please share to anyone who

UNE EN ISO 11135:2015/A1:2020 Sterilization of health-care products - Ethylene oxide - Requirements for the development, validation and routine control of a sterilization process for medical devices - Amendment 1: Revision of

Medical device software and sterilization professionals - We are proud to announce the CPD accreditation of our online course regarding validation of sterilization according to EN ISO 11135. This is important for

ANSI/AAMI/ISO 11135:2014/A1:2018; Sterilization of health-care products—Ethylene oxide—Requirements for the development, validation and routine control of a sterilization process for medical devices—Amendment 1: Revision of Annex E, Single batch release

SGS Academy United Kingdom - Introduction To Ethylene Oxide (EO) Sterilisation and ISO 11135 (On-Site)

Sterilization of health-care products—Ethylene oxide—Requirements for the development, validation and routine control of a sterilization process for medical devices—Amendment 1: Revision of Annex E, Single batch release | ANSI/AAMI/ISO 11135:2014/A1 ...

PDF) BSI Standards Publication Sterilization of health-care products — Ethylene oxide — Requirements for the development, validation and routine control of a sterilization process for medical devices | Trung Tien Tien - Academia.edu

AS ISO 11135-2002 Medical Devices - Validation and Routine Control of Ethylene Oxide Sterilization PDF | PDF | Sterilization (Microbiology) | International Organization For Standardization